
Topics in Photographic Preservation 1993, Volume 5, Article 11 (pp. 95-116)
Address: Detroit Institute of Arts, Conservation Services Laboratory, Detroit, MI 48202
The value of a photographic print depends both on its rarity as well as the condition and quality of the image. There are several methods currently being used on an experimental basis to chemically enhance photographic images. It would be useful, therefore, to be able to determine whether a photograph is truly pristine, or if it has been treated. In this study a variety of expendable photographs were treated with two of these methods: gold chloride or borohydride solutions. A number of these test prints were analyzed before and after treatment by energy-dispersive X-ray fluorescence spectrometry (XRF). These image enhancement processes and analyses will be discussed here.
Since the beginning of photography, ideas for the enhancement and restoration of fugitive images have been pursued.1 Deterioration of the image over time can be a result of defective original processing, fluctuations in temperature and humidity, as well as reaction with acidic mounts and adhesives. Though many chemical treatment methods have been described, thus far the only reliable option for saving or reclaiming the images of early printing-out photographs has been some form of copying procedure. Chemical treatment of original prints that were not previously chemically developed is very controversial, and involves issues basic to the ethical standards of practice for the field of conservation.
The desire for restoration of these images is persistent for a number of reasons:
In some cases, for example, images by artists such as Cameron and Brady, the value lost through fading can be considerable. With the incentive for image enhancement becoming stronger, several individuals have been testing chemical image enhancement processes with interesting results. One process is a type of bleach and redevelop procedure2 using gold chloride that has been published by Ross.3 The other is essentially a rediscovery of an older method published by Haist1 that uses sodium borohydride. This process is currently being used by Treadwell on faded stereoviews.4
Energy-dispersive X-ray fluorescence analysis, hereafter referred to as XRF, is an analytical tool ideally suited for examination of photographs. This technique is frequently used for analysis of metals in prints, mounts and papers. For example, it is quite easy to determine if a print contains silver, platinum, palladium, chromium or iron, and the technique may also be used to detect if a print has been toned with mercury, selenium, or other metals.5,6,7 Excellent descriptions of the theory, instrumentation, and applications of XRF can be found in undergraduate instrumental analysis texts such as Skoog's Principles of Instrumental Analysis.8 A more advanced treatment of X-ray methods can be found in Jenkins' An Introduction to X-Ray Spectrometry.9
This study was designed to answer several questions regarding faded photographic prints and their chemical intensification, such as: 1) what are the visual changes and aesthetic effects of treatment with either gold chloride or borohydride solutions; 2) is image silver being removed from the prints when these methods are used; 3) is it possible to reliably tell whether or not a print has been originally gold-toned, 4) is it possible to determine whether a print has been treated with borohydride or gold chloride solutions; and 5) can XRF analysis be used to answer some of these questions?
Preparation of Photographs. Discarded vintage photographs were selected for these experiments on the basis of their poor to very poor condition. All prints used in this study were albumen or gelatin printed-out-photographs. They were first documented photographically to provide an accurate historical record. Some prints were unmounted and residual adhesives and attachments removed, while others were treated still on their mounts.
Treatments. The prints were treated with either gold chloride or potassium borohydride solutions with minor modifications of the methods mentioned previously As both techniques are still evolving, precise formulas are not established at this time.
XRF Analyses. All energy-dispersive X-ray fluorescence analyses were performed using a Kevex 0750A Macroanalyzer - “Curator” Model, fitted with an X-ray tube having a rhodium target. Two separate secondary targets were used to analyze one spot in the maximum density area of a print and one spot in the minimum density area of the same print. Barium, in the form of a compressed pellet of barium carbonate, was used to excite the K emission lines of silver, and molybdenum, in the form of a thin wafer of molybdenum metal, was used to excite the L emission lines of gold. The X-ray tube voltage and current for analyses using the barium carbonate target were 60 KeV and a current of 2.0 mA, respectively. When using the molybdenum target, X-ray voltages of 50 or 60 KeV and currents of 0.5, 1.0 or 2.0 mA were used. Depending upon the area of maximum or minimum density available, the area was scanned with either a 2, 4 or 6mm diameter X-ray beam aperture.
Instrument reproducibility was determined by multiple measurements of the relative amount of silver at a single spot in the maximum density area of a test print and multiple measurements at one spot in the minimum density area of that same print. The variability of the amounts of silver in different spots in the dark area of a print was also measured. Variations in the integrated AgKα emission line intensities of the XRF spectrum due to repositioning of the print in the focal point of the X-ray beam were measured 5 times for one spot in the dark area of the test print. These data are presented and discussed in the Appendix.
The procedure of Ross3 was followed and consisted of bleaching faded prints in an aqueous bath of gold chloride and sodium thiocyanate to convert oxidized silver species to silver halide. After redeveloping, a second gold chloride bath serves as a toner. The print is then washed and refixed as usual.
When successful, the result is an increase in contrast, while the highlights remain clear. The range of colors in the final product can vary from lavender purple to brick red to blue-black, depending on the type of print and its individual history. As with borohydride, this process will produce some effect on all silver print materials. On collodion materials, however, the results are essentially destructive. Both the gold chloride and borohydride processes seem less effective on prints that appear to have been gold-toned originally. A low contrast, murky print will remain a weak print; it will just darken overall. For the gold process, it is even more important that a print be in good physical condition before treatment, as any stain or spot generally darkens more than the rest of the print, and purple staining can develop in damaged areas, and along the edges or breaks or tears in the emulsion. In general, the results with gelatin printed-out-photographs were poorer than with albumen prints. The mount boards were often damaged upon treatment with gold chloride. The various baths removed inks and finishes, and appeared to blanch and embrittle the paper; however, the emulsion layers tend to fare better than with borohydride treatment. Figure 1 and 2 illustrate several of these characteristics.

Although the density increased in this print with both procedures, it is an illustration of the undesirable effects that can occur
Figure 1: Sample print (IE8) treated without removing mount.
Control

Figure 2: Sample print (IE23) unmounted before treatment.
The prints were analyzed before and after treatment using XRF spectroscopy to determine the image and toning metals. A typical example of an XRF spectrum taken of the maximum density area of an albumen print using a barium carbonate secondary target is shown in Figure 3. Even though the image metal in this print is silver, a complex spectrum is seen, with peaks from diffraction (scattering), the X-ray tube target (Rh), the secondary target (Ba), and the emission lines from Fe, Ca and Pb, which are present in the primary support (mount or substrate).
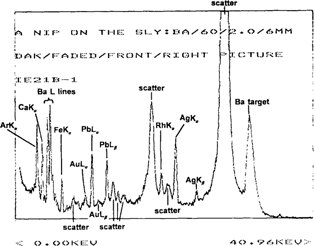
Figure 3: XRF spectrum of the maximum density area of an untreated albumen stereoview (IE21), “A Nip on the Sly”, using a barium carbonate target.
Figure 4 shows the spectrum of the minimum density area of this same print. Again, the spectrum is of the same complexity, showing peaks from scatter and the mount, but the intensity of the silver lines is noticeably lower than those emission lines from the spectrum of the maximum density area. Since the image is formed by different density areas of silver, the relative amount of silver can be determined by subtracting the XRF spectrum of the minimum density area of the image from that of the maximum density area to produce a much simpler spectrum. This results in removal of the interfering lines from other metals in the mount and/or paper support, as well as scatter peaks. The silver lines can then be integrated to give the relative amount of silver in counts/second. Subtraction of the spectrum in Figure 4 from that of Figure 3 produces the spectrum shown in Figure 5.
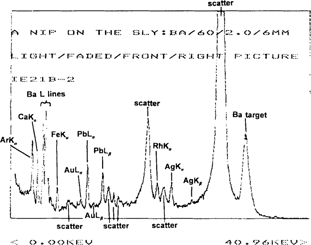
Figure 4: XRF spectrum of the minimum density area of an untreated albumen stereoview (IE21), “A Nip on the Sly”, using a barium carbonate target.
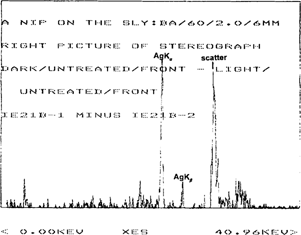
Figure 5: Subtraction spectrum of an untreated albumen stereoview (IE21), “A Nip on the Sly”, using a barium carbonate target.
Note that the gain in this spectrum is four times that of the spectra in Figures 3 and 4.
Table 1 illustrates the relative amounts of silver in counts/second before and after treatment with gold chloride solutions, as determined by subtraction.
Table 1. Amount of Silver Before and After Treatment with Gold Chloride
Print Number |
Print Description |
Amount of Ag Before Treatment (cts/sec)a |
Amount of Ag After Treatment (cts/sec)a |
Percent Decrease |
IE2 |
Van Dyke |
5.84 |
4.28 |
26.7 |
IE3 |
Minister |
7.74 |
6.70 |
13.4 |
IE6 |
Street Sceneb |
4.85 |
2.11 |
56.5 |
IE11 |
Candles |
1.23 |
0.57 |
53.7 |
IE12 |
Addie Benjamin |
1.45 |
1.14 |
21.4 |
IE19 |
House with Bush |
13.47 |
9.45 |
29.8 |
IE20 |
Group of Friends |
5.45 |
7.31 |
(34)c |
IE21 |
A Nip on the Sly |
12.24 |
8.64 |
19.4 |
IE23 |
Mutton Chops |
19.74 |
11.02 |
44.2 |
IE28 |
Doll Party |
7.74 |
4.65 |
39.9 |
IE29 |
Arc de Triomphe |
5.89 |
5.59 |
5.1 |
IE31 |
Woman with Earlocks |
4.69 |
5.07 |
(8)c |
aThese values are obtained by subtraction.
bAll prints are albumen except for the print “Street Scene”, which is a gelatin POP.
cThe values given in parentheses represent a percent increase in the amount of silver after treatment.
It can be seen from these results that, in general, a significant decrease in the amount of silver seems to occur after treatment. One explanation of this phenomenon is that the bleaching bath is acidic and contains a large excess of thiocyanate ion, which reacts with silver ions to give the complex ion, Ag(SCN)2 which is water soluble. Effective complexation of the silver ion during treatment to a readily soluble form could conceivably result in the removal of roughly 5–60 percent of the oxidized silver. In any XRF analysis, one must consider the effects of the presence of other metals in that portion of the print being irradiated.8 Thus another explanation for the decrease in silver after treatment is that it is possible that the gold metal which is deposited onto the silver particles of the image, is partially preventing penetration of the X-ray photons during analysis. This “masking” effect could cause an apparent decrease in the amount of silver being measured, but the decrease from such an effect would probably be small compared to the decrease in the amount of silver due to losses from solubilization of silver ion. Figure 6 is a bar graph plot of the data from Table 1 which shows the amount of silver before and after treatment with gold chloride solutions.
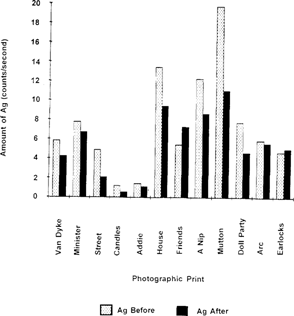
Figure 6: Relative amount of silver in photographic prints before and after treatment with gold chloride solutions.
The data in Table 1 and Figure 4 show two cases where there appears to be an increase in the amount of silver after treatment. In these two cases the increase is very slight, and it is likely that the print was scanned in slightly different dark and/or light areas of the image. The variability in the relative amounts of silver in different areas of a given print is discussed in the Appendix.
In order to determine if prints were originally gold-toned, all prints were analyzed using a molybdenum secondary target and the relative amounts of gold, given in counts/second, were determined by subtraction of the XRF spectrum in the same fashion as was done for determination of silver. Table 2 gives the relative amounts of gold before and after treatment with gold chloride solutions.
Table 2. Amount of Gold Before and After Treatment with Gold Chloride Solutions
Print Number |
Print Description |
Amount of Au Before Treatment (cts/sec)a |
Amount of Au After Treatment (cts/sec)a |
Percent Increaseb |
IE1 |
Chalotte |
0c |
13.06 |
— |
IE2 |
Van Dyke |
0c |
8.01 |
— |
IE3 |
Minister |
0c |
5.81 |
— |
IE6 |
Street Scened |
1.92 |
31.54e |
1543 |
IE11 |
Candles |
0.91 |
8.72e |
858 |
IE12 |
Addie Benjamin |
0.48 |
5.02 |
948 |
IE19 |
House with Bush |
1.27 |
12.29 |
868 |
IE20 |
Group of Friends |
7.31 |
28.55 |
291 |
IE21 |
A Nip on the Sly |
3.50 |
19.41 |
455 |
IE23 |
Mutton Chops |
0c |
21.47 |
— |
IE28 |
Doll Party |
0c |
3.67 |
— |
IE29 |
Arc de Triomphe |
1.70 |
8.69 |
— |
IE31 |
Woman with Earlocks |
1.39 |
7.20 |
— |
aThese values are obtained by subtraction.
bWhere no value is given, the percent increase could not be computed because the amount of gold before treatment was essentially zero (i.e., not detected).
cNo gold could be detected in these prints.
dAll prints are albumen except for the print “Street Scene”, which is a gelatin POP.
eAll prints are albumen except for the print “Street Scene”, which is a gelatin POP
It can be seen that several of the prints appear to have been previously gold-toned, and that in some prints, it was not possible to detect any gold. In order to say that gold is definitely present in a print, two conditions are necessary. First, both the AuLα and the AuLβ emission lines must be present in the subtraction spectrum, and second, the intensity of both of these AuL emission lines should be at least 1.5 times the intensity of the baseline noise. In all of the prints treated, regardless of the initial amount of gold present, there is a very large increase in the amount of gold after treatment. The increases range from 290 to 1500 percent or higher. Two noteworthy cases are the prints “Street Scene” (IE6) and “Candles” (IE11). For these two prints, the concentration of gold chloride used was double that called for in the intensification procedure. The percent increases in gold for these prints were found to be 1543 and 858%, respectively. The increase in the amount of gold deposited from treatment is demonstrated quite dramatically by the bar graph in Figure 1.
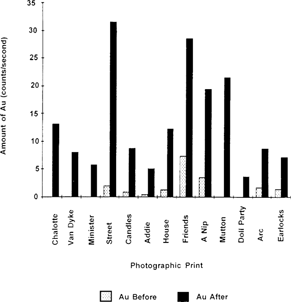
Figure 7: Amount of gold in prints before and after treatment with gold chloride solutions.
Because of the difficulty in quantitation of image metals in the absence of appropriate standards, it was decided to look at the Ag:Au ratios before and after treatment. The ratios of the amount of silver to that of gold in photographic prints, before and after treatment, are given in Table 3.
Table 3. Ag:Au Ratios Before and After Treatment with Gold Chloride Solutions
Print Number |
Print Description |
Ag:Au Ratio Before Treatment |
Ag:Au Ratio After Treatment |
Percent Decrease in Ag:Au Ratio |
IE6 |
Street Scenea |
2.53 |
0.07b |
97 |
IE11 |
Candles |
1.35 |
0.07b |
95 |
IE12 |
Addie Benjamin |
3.02 |
0.23 |
92 |
IE19 |
House with Bush |
10.6 |
0.77 |
93 |
IE20 |
Group of Friends |
0.75 |
0.16 |
79 |
IE21 |
A Nip on the Sly |
3.50 |
0.45 |
87 |
IE29 |
Arc de Triomphe |
3.46 |
0.64 |
82 |
IE31 |
Woman with Earlocks |
3.37 |
0.70 |
79 |
aAll prints are albumen except for the print “Street Scene”, which is a gelatin POP.
bThese prints were treated using a gold chloride solution that was twice the concentration called for in the procedure.
As the increase in the amount of gold far exceeds the decrease in the amount of silver, a significant decrease in the Ag:Au ratio is observed after treatment, with an average ratio of around 0.5. For “Street Scene” (IE6) and “Candles” (IE11), where the concentration of gold chloride in solution was doubled, much more gold was deposited on the print than when the normal concentration of gold chloride was used. The average Ag:Au ratio was 0.07.
Figure 8 is the bar graph of the data from Table 3 for the Ag:Au ratios before and after treatment of prints with gold chloride solutions.
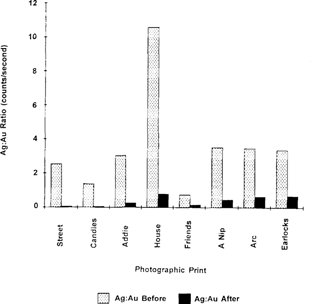
Figure 8: Ag:Au ratios of photographic prints before and after treatment with gold chloride solutions.
Treatment Procedure
This process is very simple, involving a solution of 1–2 grams of sodium or potassium borohydride in a liter of water, applied locally or by immersion. The paper base whitens from the bleaching action of the borohydride anion as the image darkens, presumably from the reduction of oxidized silver compounds back to silver metal. Conservators familiar with sodium borohydride know that this salt is quite reactive when used in aqueous solution. When the potassium salt was used, it was found to be less detrimental to the emulsion layers, especially when mixed with an ethanol/water combination, which slows down hydrogen gas evolution.10 The treatment can be effective to a degree on all silver processes, but the extent and quality of the result are impossible to predict at this point.
Characteristics of Prints After Treatment
Unpredictability is the primary drawback of the borohydride process. Image color may shift to warmer or cooler tones, and be different than that of the original. Albumen prints tend to develop a grayer tone with an unattractive density increase in the minimum density areas. Other disadvantages include occasional blistering of emulsion layers due to the evolution of hydrogen gas, and increased roughening and cracking of the emulsion surface.11 Existing spots and stains are sometimes accentuated. Handcoloring and inscriptions are often diminished or removed. Some prints rapidly darken overall to the point where the image is obscured. Existing silver mirroring is either not affected, or is made more noticeable, but foxing becomes less noticeable. Preliminary results suggest that this process may be reversible to a degree if the print is transferred to a water or water/ethanol bath. It is also possible that an oxidizing agent might reverse the process, if only partially.
There is a limited potential for improvement of a faded image, which is obviously determined by the type and amount of silver species present. A low contrast print will increase in density overall. Preliminary aging tests on treated prints indicate that they fade at the same or somewhat slower rate than an untreated original.4,11
XRF Analyses
At this time, no successful method has been developed for using X-ray fluorescence spectroscopy to determine whether or not a print has been treated with borohydride solutions. XRF did prove helpful, however, in attempting to determine whether silver is removed from a print during this treatment. The relative amounts of silver were determined before and after treatment, using subtraction spectra as described previously. These values are given in Table 4.
Table 4. Amount of Silver Before and After Treatment with Potassium Borohydride Solutions
Print Number |
Print Descriptiona |
Amount of Ag Before Treatment (counts/second)b |
Amount of Ag After Treatment (counts/second)b |
IE2 |
Van Dyke - unfixedc |
5.84 |
7.57 |
IE2 |
Van Dyke - fixedc |
5.84 |
5.97 |
IE21 |
A Nip on the Slyd |
10.35 |
14.14 |
IE23 |
Mutton Chopsc |
19.47 |
18.44 |
IE27 |
Family Theaterd |
11.83 |
11.81 |
aAll prints were albumen POP.
bRelative amounts of silver were determined by subtraction.
cThese prints were not gold-toned.
dThese prints were gold-toned.
In general, there is little or no change greater than the experimental error in the amount of silver in prints before and after treatment with borohydride solutions. This is not an unexpected result, since the borohydride reduces oxidized silver in any form to silver metal; thus silver is not being removed from the print by any chemical or physical means. XRF generally detects an element regardless of its chemical form, whether it be the ion or the reduced species.
In those cases where there appears to be an increase in the amount of silver, it was necessary to scan different parts of the image in the dark and/or light areas before and after treatment. It is presently impossible to detect if a print has been treated with borohydride based solely on the analysis of silver before and after treatment. Work continues to search for a way to detect if a print has previously been treated. It may be possible to measure the amount of sulfur present before and after treatment, as a possible indicator of prior chemical image enhancement.12
Figure 9 shows a bar graph representing the amount of silver found in the prints before and after treatment of the prints with potassium borohydride.
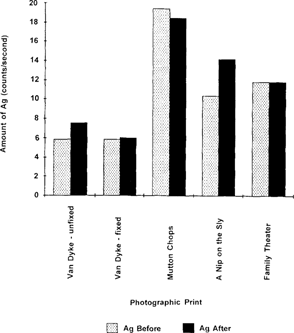
Figure 9: Amount of silver before and after treatment of the prints with potassium borohydride solutions.
For either of these processes, results varied wildly by the type of print, its condition and processing, the amount of time spent in solution, and the temperature of the solution baths used. In general, the gold process produced warmer tones than borohydride, and more dramatic results. What is seductive is the occasional extremely good result. It must be remembered that many curators and conservators advocate no chemical treatment of vintage printed-out materials, including water, and that certainly with the gold process, the size and composition of the image forming particles are changed irrevocably.
Because of the extreme variability of the results, even with further refinement these chemical image enhancement procedures could only be considered useful to retrieve documentary information from essentially valueless artifacts. Thorough aging studies of treated prints still need to be done. Also critical is whether or not it is possible to detect a print that has been chemically enhanced, and how the value of the print might be affected. Visually, a treated print might appear to have a surface slightly rougher than another of its type. The print color may be unusual when compared to others of the same period. Provenance is very important in evaluating a work of art for a purchase, and if the history of the print is known, doubts about its appearance might be put to rest.
Would an extremely faded image be considered valuable once again if successfully treated with a process such as these? Making comparisons to other areas of conservation provides some perspective. An old master painting can seem very different when aged varnish and overpaint are removed. In another example, the problem of white lead pigments blackened by atmospheric sulfur can be dealt with by using hydrogen peroxide to convert the black sulfide to a stable lead sulfate, a form completely different than the original basic lead carbonate. However, some curators prefer the stained version, comparing it to the natural patinas on bronzes. When corrosion problems threatened a large outdoor sculpture at The Detroit Institute of Arts, a different, more durable paint was used to better protect the piece, even though the artist's original coating was still available from the manufacturer.13
One must use optimum conditions when performing XRF analyses. It is important, especially when looking for small amounts of toning metals, such as gold, to use both barium and molybdenum secondary targets. An energy just slightly higher than the excitation energy of the element being analyzed should be used. If this energy is too high, then ionization and not fluorescence will occur. This will result in decreased line intensities for those metals requiring a lower excitation energy. For example, the barium secondary target is useful for near optimal excitation of Ag, Pd and Sn. Though it may still be possible to see metals such as Pb, Au, Fe, Cu, Co, Cr, Zn or Ni using a barium secondary target, much larger emission line intensities can be obtained using a Mo secondary target instead. One must also be careful to position the print carefully in the focal point of the X-ray beam to reduce the variability in measurement of the amount of metal, as described in the Appendix. One must also use caution when interpreting XRF data. To say for certain that gold is present in a print, both the AuLα and the AuLβ emission lines must appear in the subtraction spectrum and should be at least 1.5 times the intensity of the baseline noise. The advantage of using XRF for the analysis of photographic prints is that it is a both non-destructive and non-invasive technique.
There are many issues which have been raised by this research and questions which need to be addressed. For example, although the use of XRF analysis for determining whether or not a print has been enhanced with gold shows promise, more samples need to be evaluated. Furthermore, if the idea of chemical image enhancement of faded images becomes more legitimate, it would be interesting to explore methods for predicting which prints would react in a favorable way, and to determine the circumstances most likely to give a good result. In addition, evaluation of the amount of sulfur present before and after treatment could prove very informative, and might provide a means of detecting whether borohydride has been used.15
The authors wish to thank the National Endowment for the Arts and the Andrew W. Mellon Foundation for their support. The authors are also indebted to their many colleagues who generously shared their knowledge and expertise.
1. Haist, G.; Modern Photographic Processes, Volume 2, John Wiley and Sons, New York, NY, 1979, pp. 39–49, and references cited therein.
2. Hendriks, K.; Thurgood, B.; Iraci, J.; Lesser, B.; Hill, G.; Fundamentals of Photographic Conservation: A Study Guide, Lugas Publications, Toronto, Ontario, Canada, 1991, pp. 388–395.
3. Ross, L.; “Chemical Restoration of Albumen and Gelatin Printing-Out-Paper Prints”, Journal of Imaging Science, 1986, 36 (1), 56–59.
4. Treadwell, T.K.; “Intensification of Photo Images by the Sodium Borohydride Process”, National Stereoscopic Association Information Service, unpublished work.
5. S. Rempel “Qualitative Energy X-Ray Fluorescence Examination of Historic Photographic Artifacts”, presented to the Photographic Materials Group of AIC, Charlestown, SC, March 1986.
6. Lee, W.E.; Wood, B; Drago, F.J.; “Toner Treatments for Photographic Images to Enhance Image Stability”, Journal of Imaging Technology, 1984, 10 (3), 119–126.
7. Enyeart, J.L., Anderson, A.B., Perron, S.J., Rollins, D.K., Fernando, Q.; “Non-Destructive Elemental Analysis of Photographic Paper and Emulsions by X-ray Fluorescence Spectroscopy”, History of Photography, 1983, 7, 99–112.
8. Skoog, D.A.; Principles of instrumental Analysis, 3rd Edition, Saunders College Publishing,Philadelphia, PA, 1985, pp. 456–485.
9. Jenkins R.; An Introduction to X-Ray Spectrometry, Heyden and Son, Ltd., London, England, 1976.
10. D. Nishimura of the Image Permanence Institute, private communication.
11. Stodulski, L.P.; Baas, V; Severson, D; “A Preliminary Investigation into the Effects of Aqueous Sodium Borohydride Solution on Silver-Based Photographic Materials”, presented to the Photographic Materials Group of AIC, New Orleans, LA, June 1988.
12. K. Hendriks of the National Archives of Canada, private communication.
13. Unpublished lecture, C.Forsythe, Midwestern Regional Conservation Guild, October 1986.
In order to assess the validity of the XRF data, answers to three important questions were required: 1) does the instrument give reproducible data for replicate analyses on a particular area on the print; 2) how does the amount of silver vary within a homogeneous dark or light area on a print; and 3) how does repositioning of a print in the X-ray beam affect quantitation of the amount of silver in the print?
To assess instrument reproducibility, several scans of each of two areas on an image were done under the same instrumental conditions and the relative amounts of silver in counts/second were determined. These data are given in Table A. Integration of the AgKα emission line for the dark area of the image agreed within approximately 3%, and that of the light area within 1.3%. Thus any change in the integration of silver after treatment of less than 3 percent would not be a significant change.
Table A. Variation of Readings for Silver Within the Same Dark Area or Light Area of a Print Image
Image Area |
Integration of AgKα Peak (counts/second) |
Percent Deviation |
dark |
25.94 |
0.61 |
dark |
26.74 |
2.45 |
dark |
25.68 |
1.61 |
dark |
29.04 |
0.23 |
light |
18.58 |
1.34 |
light |
18.79 |
0.23 |
light |
19.02 |
1.00 |
light |
18.94 |
0.57 |
Although a maximum density area of an image may visually appear to be quite homogeneous, that does not necessarily mean that the amount of silver is uniform throughout. To test this hypothesis, XRF analyses were done on 4 different maximum density areas and 4 different minimum density areas that were visually uniform in appearance. The variation in the amounts of silver in counts/second is given in Table B. For the maximum density areas, the variation of the amount of silver ranged from 3–17%, while that of the minimum density areas ranged from 5–11%. Thus unless XRF spectra were taken of exactly the same spot on a print, a change of less than 15% is probably not significant, but a change of greater than 20% can be considered real and significant.
Table B. Variation of Readings for Silver in Slightly Different Dark Area or Light Area of a Print Image
Image Area |
Integration of AgKα Peak (counts/second) |
Percent Deviation |
dark |
32.80 |
17.4 |
dark |
28.80 |
3.1 |
dark |
24.24 |
13.3 |
dark |
25.94 |
7.2 |
light |
18.94 |
6.9 |
light |
18.64 |
8.4 |
light |
22.51 |
10.7 |
light |
21.28 |
4.6 |
Another source of experimental error comes from scanning the print at exactly the same spot, but at slightly different distances of the X-ray source from the print surface. This, of course, could occur each time the print is repositioned in the focal point of the instrument beam. The variation in the apparent amount of silver in a particular area of the image upon repositioning of the print is given in Table C.
Table C. The Variation of the Amount of Silver upon Repositioning of the Print
Position |
Amount of Silver (counts/second) |
Percent Deviation |
1 |
27.41 |
6.8 |
2 |
31.02 |
5.5 |
3 |
28.90 |
1.7 |
4 |
31.04 |
5.6 |
5 |
28.62 |
2.6 |
Scanning of the same area on the print, but repositioning the print and realigning the beam focal point, results in a percent deviation of less than 7%. Thus we can be confident that changes in less than 10% in the counts/second is not an error from repositioning of the print in the X-ray beam.