Topics in Photographic Preservation 1999, Volume 8, Article 10 (pp. 67-70)
In order to create great image density and image quality in any wet plate process, the silver solution must coat and adhere to the collodion surface. This poses a problem, as the collodion will repel the water in solution because the collodion is hydrophobic. However as a silver bath in any wet plate collodion process is used, the ability for the silver solution to adhere and coat the collodion more evenly increases. This is due to the increase in wetablility between the silver solution and the collodion surface. Which simply means that as the silver solution is used the silver solution begins to become more collodion like. This is due to the fact that as the collodion plates are placed in the silver solution the collodion begins to dissolve into the silver bath, thus becoming more like collodion.
A brief overview of the Ambrotype process is this: a solution of salted collodion is made and allowed to sit for several days, it is then poured on glass, dipped in a solution of six percent silver nitrate, exposed, stopped, fixed and washed. The image is then allowed to dry and the glass is then backed usually with black lacquer and placed in a metal or wooden frame. The ambrotype process, like all photographic processes has its tricks of the trade and certain key aspects behind it, which will become useful in the creation of the final image. Most ambrotypist will tell you the key to creating the perfect ambrotype is to leave a coated glass plate in the silver nitrate bath over night. (1) Why is that? Well, there are several reasons, many think the iodine or halide in the collodion will pre-sensitize the silver bath. Others believe the collodion, along with the organic materials, in the silver bath will act as a wetting agent in the solution. Lastly a final hypothesis on the pollution of the silver bath is that as the collodion is allowed to sit in the solution, the silver and iodine form light sensitive silver halides, which float around in solution and redeposit on the next glass plate. Thus creating floating density in the silver bath. All solutions seem to have some validity however the idea, which held ground in the investigation, was the idea of the organic materials acting as a wetting agent. The image density is usually very thin, or light especially when using a fresh silver bath, however as the silver bath becomes polluted with collodion, ether, and alcohol, the density of the images becomes darker. This is why most professional ambrotypist will leave a coated glass plate in the silver bath overnight. The collodion will eventually precipitate into solution and result in a polluted silver bath. This is where the investigation will begin.
To perform this examination clearly, a device called a contact angle-measuring device was used. The machine consisted of a flat platform placed on an adjustable post, whereby the platform could be moved up, down, left, and right. There is one lens in the front of the platform, which is directed towards the platform and illuminated by a green light. Inside the lens is a protractor with moving lines to measure the angles. The angle is measured from the horizontal line up to the inside edge of the bubble. Each silver bath solution in the normal course of the silver bath were taken and measured on a collodion surface. Starting with fresh distilled water to give a reference point. Then the fresh silver bath consisting of distilled water with silver nitrate. Next the two test silver baths consist of one with collodion, and the other with collodion ether and alcohol. The final bath tested was a silver bath with all pollutants, including collodion, ether, alcohol, and iodine. The last bath represents the silver bath, which has been used several times. All five solutions were measured individually.
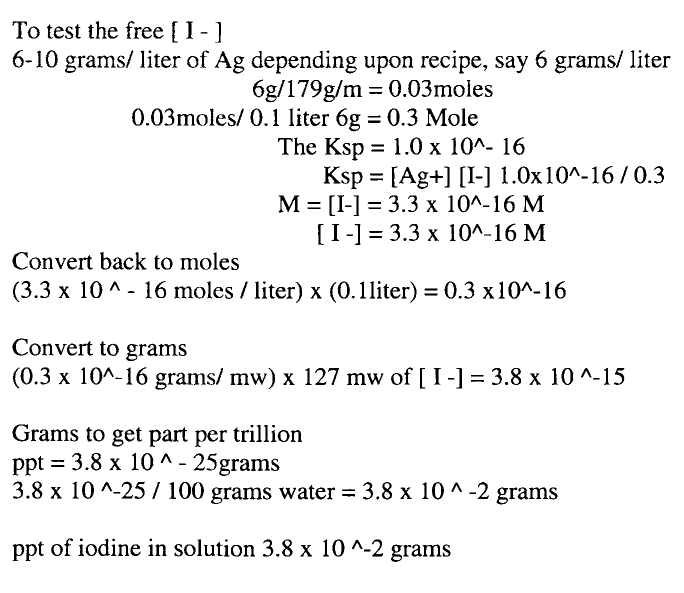
The idea of the iodine or bromine acting as a pre-sensitizer to the silver bath is far fetched, as there is only approximately 3.8 × 10∧-15th grams in solution. (Appendix A) Thus ruling out that idea, along with the idea of silver and iodine forming light sensitive silver halides, which float in solution and redeposit on the next glass, as the amount of silver halides in solution is miniscule. Which leads us to the final hypothesis of the collodion, ether, and alcohol acting as a wetting agent. When liquids such as water, silver solutions, developer, stop, and fixer solutions come into contact with collodion, glass, plastic, etc. the surface energy of each will regulate the outcome. The surface energy will create what happens in the interfacial region or boundary where one phase (liquid) ends and another (solid) begins. (Thompson. 176) A liquid adopts a shape that reduces its surface area to a minimum because it is the most efficient way of packing molecules with their neighbors. Droplets of a liquid therefore tend to be spherical unless distorted by gravitational or inter-phase forces. Common examples are to be seen in raindrops and the drops from a tap. The internal forces acting between molecules pull them together into shapes of the lowest surface area. These are called cohesive forces and the phenomenon is one of cohesion. The forces responsible are those intermolecular, van der Waals and polar forces that are responsible for holding materials together. They are strongest in solids and weakest in vapors and gases. (Thompson, 176) When a liquid comes into contact with another phase such as a solid, forces of attraction will usually be set up between the two phases, the strength of which determine what happens at the interface. If the forces between molecules in the solid and the liquid interfaces are negligible, the spherical shape of the liquid droplet is largely unaltered. Water droplets on a greasy surface, like collodion on a glass plate is a very good example of this. The surface energy of the collodion and the surface energy of the water are so different that it will cause the water the bead up on the surface. On the other hand, if the intermolecular forces of attraction are very strong, the liquid will be pulled onto the surface and flatten out. When the forces cause the liquid to spread, wetting is said to occur and conversely, non-wetting is the condition where zero or only partial spreading takes place. In order to measure the amount of wetting taking place at the interface, the contact angle between the liquid and solid is measured. Depending upon the degree to which the angle is wetted depends upon the surface energies of the two compounds. The tables below found in “Printing Materials: Science and Technology” displays the surface tensions of water and some organic solvents at 293K. Also the surface energies of glass and some polymer films.
SOLVENT SURFACE TENSION (mN/m)
Water |
72.6 |
Methanol |
22.6 |
Ethanol |
22.3 |
Propan-2-ol |
21.4 |
Ethyl ethanoate |
23.9 |
MATERIAL UNTREATED (mN/m)
Low density polyethene |
31 |
High density polyethene |
31 |
Polyester |
43 |
Nylon 6,6 |
46 |
Polyvinyl chloride |
41.9 |
Polydimethyl siloxane |
19.8 |
Glass |
75 |
Both glass and water have the highest surface energies of their categories, which indicates that pure water will wet glass, however when the water is placed on polyethene the differences in surface energies will create non-wetting. The polyethene can be compared to collodion in that the two compounds are both hydrophobic compounds and the surface energies can be assumed comparable. Therefore it makes sense that when the glass coated with collodion is placed into the solution of silver nitrate, made primarily of water, the water will trap the silver through non-wetting and not allow the silver to pass through to the iodine trapped in collodion. Now, to break the barriers, or decrease the surface energies, the energy of one solution must be decreased. This conveniently happens when the ether and alcohol precipitates from the collodion through continued use of the silver bath, or when leaving a coated glass plate in the solution overnight. To prove this phenomenon the contact angles of the various solutions used in ambrotypes were measured.
SOLUTIONS CONTACT ANGLE
Distilled water |
40 |
Distilled water with silver nitrate (AgNO3) |
36.2 |
Distilled water with collodion (2g) |
31 |
Distilled water with collodion, ether, alcohol |
25.1 |
Distilled water with pollutants |
21.3 |
Although the surface energies of each solution was not measured the contact angle is directly related, because the smaller the contact angle the less the differences are in surface energies. Therefor the surface energy of the water was decreased based on how much ether, alcohol, collodion, and other pollutants were introduced into solution. As the contact angle is decreased it creates an easier barrier for the silver in solution to pass though to the iodine or bromine in collodion to create greater density. Thus proving the original hypothesis that as the silver bath is used and polluted, the density on the silver plates will increase, due to the organic materials acting as a wetting agent.
