Topics in Photographic Preservation 2009, Volume 13, Article 16 (pp. 100-109)
Presented at the 2009 PMG Winter Meeting in Tucson, Arizona
Twentieth-century stabilized prints are silver-gelatin photographs that have been subjected to a two-bath process: activation of an incorporated developer and stabilization of the development process. Unlike traditionally processed silver gelatin prints, stabilized prints have not been fixed or washed and were originally intended to provide quick, high- quality images for proofing. These prints are vulnerable to darkening, staining, fading or complete image loss and have been observed to transfer stains to adjacent objects. Accurate identification of stabilized photographs will increase understanding of this unique process and lead to better informed conservation and preservation decisions.
This paper provides a basic description of the stabilization process and presents results from X-ray fluorescence spectrometric analysis of a group of Diane Arbus photographs suspected to have been printed using a stabilization process and a corresponding group processed traditionally. Results of the XRF analysis indicate that the elemental composition of the stabilized processed photographs differs from traditionally processed silver gelatin photographs in that silver is present in the highlights and non-image areas and higher than normal amounts of halides remain in the print. The presence of unreacted halides and elevated levels of silver overall are consistent with a photograph that has not been fixed or washed and may provide a set of characteristics useful for identification of stabilized silver gelatin photographs.
In January 2002, a group of 22 black and white fiber base photographs by Diane Arbus, on 11 × 14 inch paper, arrived for examination and possible treatment at The Conservation Center for Art and Historic Artifacts in Philadelphia. They were acquired by The New Jersey State Museum in 1979 and have been housed in 4-ply alkaline window mats in climate controlled storage since acquisition. On the back of the photographs there are notations including occasional handwritten felt marker inscriptions of “Eliminated” or “OK” in addition to numbers apparently written in pencil. Neil Selkirk, the exclusive printer of Arbus’ photographs since her death in 1971, examined the 22 prints at CCAHA on August 25th 2009, and positively identified the numbers on the back as having been written by him to identify the images at the time he made the prints in 1972. These images are part of Arbus’ Untitled series. The photographs were taken by Arbus from 1969 to 1971 at various residences for developmentally handicapped adults throughout New Jersey.
Preliminary research on these photographs began in 2002 at The Conservation Center for Art and Historic Artifacts in Philadelphia. X-ray fluorescence spectrometry was conducted in 2008 at The Museum of Modern Art in NY with the assistance of Chris McGlinchey, Ana Martins and Lee Ann Daffner.
Figure 1 Reprinted with permission from CCAHA
As mentioned above, the photographs from the New Jersey State Museum have never been exhibited and have been stored under climate controlled conditions since their acquisition in 1979. Nonetheless, the museum has noticed significant deterioration over time. At first look, the photographs appear to be in excellent condition. The single-weight, fiber base paper supports have minor handling creases but no tears or losses and although many of the images have a flat tonality, the silver image material is in good condition with no silver mirroring or fading visible. However, when the photographs are removed from the mats, significant brown staining can be seen on the margins and versos of all the photographs (Figure 1) and the interiors of the mats. (Figure 2) In fact, significant discoloration is found wherever the mat and photograph are in direct contact with one another. Discoloration can also be seen on the versos of the back mats in a shape and size corresponding with that of the window opening of the mat stored beneath it in the stack. (Figure 3) This appears to be the only case where the brown staining is not the result of direct contact with the photographs.
The deterioration pattern is not one commonly observed with conventionally processed silver gelatin photographs and the typical causes of discoloration can be easily eliminated; the photographs have not received significant light exposure and pH and fiber analysis indicated good quality mat board and photographic paper. Rather, the dark brown staining of the photographs and adjacent materials is similar to that observed with stabilized silver gelatin photographs. Although not necessarily representative of the known body of Diane Arbus’ work, exhibitions such as “Family Album” and “Revelations” have included stabilized prints and have made reference to her use of a Fotorite stabilizing processor and chemistry as a means of proofing prints before creating final enlargements. Subsequent conversations with Neil Selkirk have confirmed that Arbus owned a Fotorite processor (which is still in the possession of the estate) and used stabilized prints when making contact sheets and 8 × 10 proofs. The image quality and tone of the New Jersey State Museum photographs, however, are exceptionally well preserved when compared to other stabilized photographs of this era and are printed on 11 x14 instead of the standard 8x10 paper.
Figure 2 Reprinted with permission from CCAHA
Stabilization can be defined as a rapid two-step photographic process in which an alkaline solution (the developer activator) is applied to a developer impregnated paper followed by a slightly acidic stabilizer solution. By definition, these images are not fixed and washed, merely stabilized to reduce sensitivity to light. In 1950, H.D. Russell,
E.C. Yackel and J.S. Bruce defined stabilization as “fixation without washing to make the unexposed and undeveloped silver halides relatively inert to heat, light, humidity and atmospheric gases". (Russell, Yackel, Bruce 1950; Haist 1979, 2000) Since the process does not include a fixing or washing step, all the residual chemistry is retained in the print. “Stabilization processing” is an umbrella term encompassing six major systems; thiourea, thiosulfate, thiocyanate, thiol, iodine and heat or dry stabilization. Although thousands of patents for stabilizing processes were issued, thiourea, thiosulfate, and thiocyanate-based processes seem to have enjoyed the widest use. There is some indication that Diane Arbus used both the Kodak Ektamatic stabilization paper and chemistry (a thiocyanate-based system) and the Agfa Fotorite stabilization system.
Figure 3 Reprinted with permission from CCAHA
Stabilized processed photographs were a 20th century commercial invention prepared for the mass market and intended to be quick, simple and temporary. Although many stabilization processes were patented during and after WWII, the process reached its zenith during the 60s, 70s and early 80s when the market then turned toward computers, copiers and printers to create high quality pictures quickly and easily. Besides speed of production, the stabilization process had the advantage of being simple enough for anyone to use without prior knowledge of photochemistry. Premixed bottles of chemistry and a table top processor requiring no water made the process extremely portable and adaptable to a variety of circumstances. The stabilization process found wide use in the newspaper, military, medical and scientific fields - anywhere that speed of image production was more important than long-term image stability. (Sturge 1977)
In general, the chemical agents used for conventional processing were also used for stabilization processing. However, while conventional silver-based processing includes developing, stop bath, fixing and washing steps, stabilization processing contains only two steps: activation of the developer incorporated in the paper and stabilization of the silver image. In conventional processing, developers contain a hydroxide ion activator and a reducing agent, such as hydroquinone, to reduce silver halide to silver metal. In stabilization processing, a hydroquinone reducing agent is already incorporated into the paper. This allows the development process to begin immediately when the paper is immersed in an alkaline activating solution such as potassium bromide or sodium hydroxide. The stop and fixing baths are omitted in stabilization processing and the print proceeds directly from a slightly alkaline developing solution to a slightly acidic stabilizing solution. The stabilizing bath complexes the silver halide instead of removing it through fixing and washing, converting the undeveloped silver halides to colorless compounds, such as silver salts of thiosulfate, thiocyanate, or thiourea which are more or less stable to light. (Sturge 1977) As a result, the process leaves both silver compounds and residual chemistry in the print creating a photograph that is much less permanent than conventionally fixed and washed images. For better permanence, stabilized prints could be traditionally fixed, washed and toned shortly following processing
In order to decrease processing times, silver gelatin stabilized papers were manufactured with a developer already in the emulsion ("developer- incorporated"). Immersion and application times of less than 10 seconds were recommended to prevent excessive absorption and a tabletop roller transport processor could turn out a dry-to-dry 8 × 10 photograph in approximately 10 seconds (Sturge 1977). Although manufacturers usually included directions for both tray and machine processing, machine processing was recommended because of the precise timing and temperatures needed for proper pickup of solutions. Papers intended for stabilization processing were commercially available, but traditional photographic paper could also be processed with stabilization chemistry.
Incorporated developers have been known to diffuse through stacks of resin-coated papers causing brown staining in processed prints, especially under conditions of high humidity or temperature. (Wilhelm 1993) In one of his patents, Edward Yackel stated, “I have found...developing agents such as ... hydroquinone...do not remain in the emulsion layer during long periods of keeping. Instead, it wanders throughout the gelatin and waterproofing layer of a material..and from one piece of material to another when the two are in close contact. This wandering of the developing agent results in undesirable stain.” (Yackel 1947) Since residual hydroquinone is washed out during traditional photographic processing, brown staining is less of a concern in fiber base photographs. In stabilization processing however, the developing agents and other processing chemistry remain in the print. It is quite possible that the brown discoloration on the mats and photographs is partially the result of “wandering developer".
Although some stabilized photographic processes can be characterized by an overall warm color, flat tonality, fading or discoloration, a well preserved print may not exhibit any of these characteristics. In addition, the wide variety of stabilization processes often makes it extremely difficult to discern stabilized prints from other photographic or graphic reproduction processes. Improper identification could lead to unwise decisions regarding preservation or treatment, ultimately endangering the prints and the collection items surrounding them. Elemental analysis using X-ray fluorescence spectrometry (XRF) can be used to detect the presence of inorganic materials in photographs such as silver, halides and sulfur, theoretically providing an identifying fingerprint for each process. In a traditionally processed photograph, one would expect to find silver only in the dark and mid-tones where the image is formed by the presence of metallic silver. In non-image areas or highlights, where unreacted silver has been removed by fixing and washing, one would not expect silver to be present. Similarly, although trace amounts of halides can be detected in conventionally processed photographs, the majority of light sensitive bromides or chlorides are removed during the fixing and washing process. Higher than normal amounts of halides in the print as well as the presence of silver in non-image areas could indicate that a photograph was stabilized processed.
Four photographs from the New Jersey State Museum’s Untitled series and four identical, but traditionally processed images from the collection at The Museum of Modern Art were analyzed side by side in the MoMA Conservation Department. Chris McGlinchey and Ana Martins from the Conservation Science department at MoMA performed the analysis and data interpretation.
The photographs were analyzed using the Tracer III-V portable XRF manufactured by Bruker (nee Keymaster) with a Rhenium target and silicon PIN detector. Data was collected under two separate conditions: 40 keV and 4 micro amps with a copper, titanium aluminum filter set (for high z excitation) and 15 keV and 15 micro amps with no filtration for low Z excitation. The former relied on bremstrahlung for excitation and the latter the rhenium L lines. In this study (optimized for both tube and filtering conditions), iron and below was considered low z.
The photographs were photocornered into precut window mats with a Coroplast backing which would not interfere with the spectrometric readings. For each of the photographs, three readings were taken from an area of minimum density (D-min), two areas of maximum density (D-max) and one non-image area in the margin. An additional reading was taken at the top right corner of the stabilized prints where discoloration was present (Figures 4, 5, 6, 7). Untitled #6 corresponds with MoMA 224.2004; Untitled #8 corresponds with MoMA 331.1972; Untitled #5 corresponds with MoMA 224.2004; Untitled #14 corresponds with MoMA 335.1972.
Figure 4: Untitled (6) 1970-71 Copyright ©1972 The Estate of Diane Arbus, LLC. Courtesy of the New Jersey State Museum, Trenton
Figure 5: Untitled (8) 1970-71 Copyright ©1972 The Estate of Diane Arbus, LLC. Courtesy of the New Jersey State Museum, Trenton
Figure 6: Untitled (5) 1970-71 Copyright ©1972 The Estate of Diane Arbus, LLC. Courtesy of the New Jersey State Museum, Trenton
Figure 7: Untitled (14) 1970-71 Copyright ©1972 The Estate of Diane Arbus, LLC. Courtesy of the New Jersey State Museum, Trenton
Graph 1 shows the amount of residual silver in the D-min locations of both the stabilized and traditionally processed prints. As expected, readings from the traditionally processed prints from the MoMA collection followed the convention of little or no silver in the D- min and non-image areas. In fact, silver readings from the D-min and margin areas are comparable to readings from the matboard and the Coroplast. The New Jersey State Museum photographs, however, contain a greater concentration of silver throughout the entire photograph including the white non-image border areas.
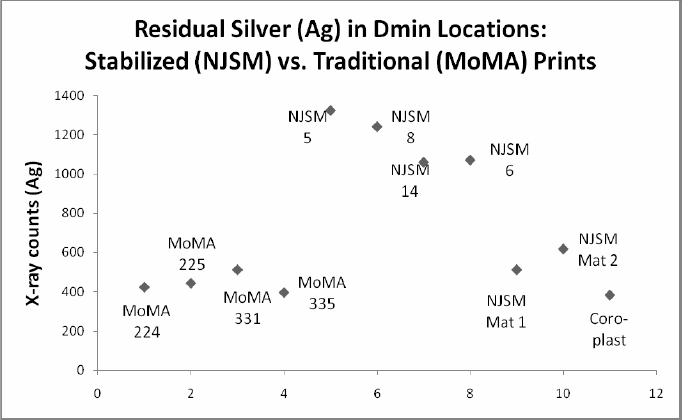
Graph 1: X-ray counts showing elevated levels for silver in Dmin locations of NJSM photographs compared to low (background) levels for Dmin in MoMA prints, NJSM mat and Coroplast samples.
Graph 2 compares the XRF spectra in D-min locations of Untitled #5 and its counterpart MoMA 224. Note the silver peak present in the New Jersey State Museum print but non- existent in the MoMA print.
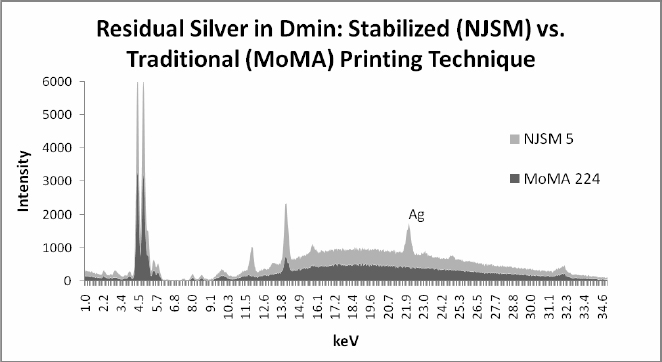
Graph 2: X-ray Fluorescence (XRF) spectra of Dmin from the same image printed two different ways: stabilized print at top (NJSM 5) showing residual silver (Ag) and no detectable Ag (only background radiation) in MoMA traditional print. XRF location of ‘MoMA 224’ and ‘NJSM 5’ are approximately identical.
Readings for the presence of halides also confirmed expectations for both the stabilized prints and traditional prints. Extremely small amounts of residual halide are found in the traditionally processed prints while the stabilized prints contain significantly higher amounts of bromine. The presence of unreacted halides and elevated levels of silver overall are consistent with a photograph that has not been fixed or washed.
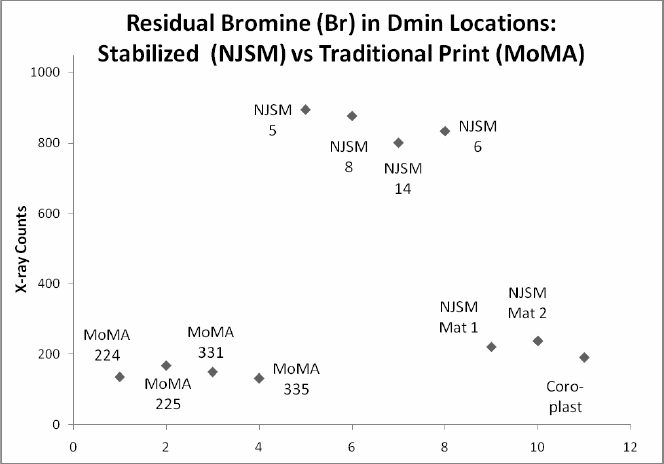
Figure 3: X-ray counts showing elevated levels for Bromine in Dmin locations of NJSM photographs compared to low (background) levels for Dmin in MoMA prints, NJSM mat and Coroplast samples. Experimental conditions: unfiltered Rhenium excitation, 15 keV voltage and 15 microamps current.
Elevated levels of sulfur and cadmium were also found in the New Jersey State Museum photographs. Significantly higher amounts of sulfur may be the result of retained chemistry in the print, especially if a thiosulfate-based stabilizing system was used. Although the difference is not significant, the stained areas of the New Jersey State Museum prints contain slightly higher levels of sulfur than in the unstained non-image areas. The source of cadmium in both the traditional and stabilized papers may be attributed to the addition of cadmium during the paper manufacturing process in order to provide a warm image tone. Excess sulfur and cadmium may also be an indication that a print was never washed after processing to remove residual chemistry and manufacturing additives.
XRF analysis indicates that the elemental composition of the New Jersey State Museum stabilized processed photographs differs from traditionally processed silver gelatin photographs. The presence of silver in the highlights and non-image areas is consistent with a print that has not been fixed and washed since the silver remains throughout the print. The presence of higher than normal amounts of bromine (or halides in general) is also consistent with stabilization processing since both reacted and unreacted halides remain in the print. It should be assumed that high amounts of unreacted halides in the prints indicate a tendency toward continued light sensitivity and exhibition of the originals is not recommended. Elevated levels of sulfur and cadmium seem to further indicate that the prints were not washed following processing.
A clear mechanism for discoloration could not be discerned from the XRF analysis. It could be a result of alkaline interactions, developer diffusion, oxidation of residual chemistry or a combination of many causes. Understanding the mechanism could be helpful in preventing or slowing down the progress of deterioration of stabilized prints and adjacent collection materials. Using neutral pH, all-rag matboard, interleaving paper or folders is a simple, non-invasive way to eliminate one of these variables. Since increased temperature, humidity and pH can cause deterioration of the excess stabilizing chemistry, storage environments should be carefully monitored.
Many thanks to Mary Schobert of CCAHA and Margaret O’Reilly of the New Jersey State Museum. Thanks to Lee Ann Daffner, Chris McGlinchey, Ana Martins, Rosina Herrera and Eva Respini at The Museum of Modern Art and Lisa Barro, Nora Kennedy and Jeff Rosenheim at the Metropolitan Museum of Art. Special appreciation to Doon Arbus, Neil Selkirk, the National Endowment for the Arts, the Photographic Materials Group membership, the Photographic Materials Group Professional Development Stipend and The New York Public Library.
Haist, Grant. 1979, 2000. Modern Photographic Processing. V2. Okemos, MI: Haist Press.
Russel, H.D, Yackel, E.C, Bruce, J.S. October 1950. Stabilization Processing of Films and Papers, PSA Journal, Photographic Science and Technique, Volume 16B.
Sturge, John, ed. 1977. Neblette’s Handbook of Photography and Reprography Materials, Processes and Systems. Seventh Edition. New York:Van Nostrand Reinhold Company
Wilhelm, Henry. 1993. The Permanence and Care of Color Photographs: Traditional and Digital Color Prints, Color Negatives, Slides, and Motion Pictures. Grinnell, Iowa: Preservation Publishing Company.